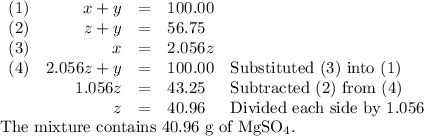
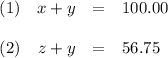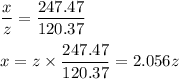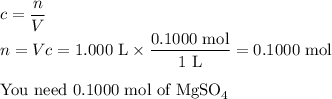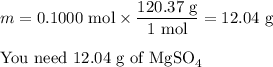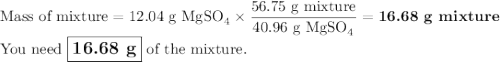Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O). You want to make a standard solution of magnesium ion, and this is the only sample of a magnesium salt you have. To determine the amount of magnesium salt in the mixture, you heat 100.00 g to drive off the water of hydration and find that the anhydrous mixture has a mass of 56.75 g. How many grams of the original salt mixture must you add to 1.000 L of water to make a 0.100 M solution of Mg²⁺ ion? g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O...
Questions

Biology, 08.07.2019 01:30

Geography, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30


Mathematics, 08.07.2019 01:30



Chemistry, 08.07.2019 01:30


English, 08.07.2019 01:30


History, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30

Arts, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30


Mathematics, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30