

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
Classify the substances according to the strongest solute-solvent interaction that will occur betwee...
Questions



Mathematics, 23.11.2020 23:10

Physics, 23.11.2020 23:10


Mathematics, 23.11.2020 23:10

Biology, 23.11.2020 23:10


World Languages, 23.11.2020 23:10


Advanced Placement (AP), 23.11.2020 23:10

Mathematics, 23.11.2020 23:10



German, 23.11.2020 23:10

Health, 23.11.2020 23:10



World Languages, 23.11.2020 23:10

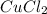 ,
, 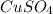 ,
, 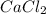 , NaCl etc are all the molecules that will show ion-dipole interactions.
, NaCl etc are all the molecules that will show ion-dipole interactions.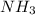 ,
, 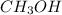 etc are the molecules which show dipole-dipole interactions.
etc are the molecules which show dipole-dipole interactions.

