
Chemistry, 27.02.2020 23:53 hoytkeke6776
A solution with a total volume of 1000.0 mL contains 37.1 g Mg(NO3)2. If you remove 20.0 mL of this solution and then dilute this 20.0 mL sample with water until the new volume equals 500.0 mL, what is the concentration of Mg 2 ion in the 500.0 mL of solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
A solution with a total volume of 1000.0 mL contains 37.1 g Mg(NO3)2. If you remove 20.0 mL of this...
Questions

Geography, 02.06.2021 17:50


English, 02.06.2021 17:50



Mathematics, 02.06.2021 17:50

English, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50



Mathematics, 02.06.2021 17:50

Mathematics, 02.06.2021 17:50



Mathematics, 02.06.2021 17:50




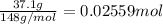
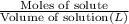
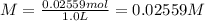
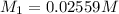
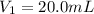
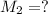
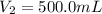
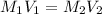 (dilution )
(dilution )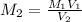
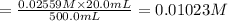
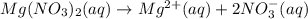
![[Mg^{2+}]=1\times 0.01023 M=0.01023 M](/tpl/images/0527/3801/31d0c.png)


