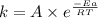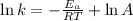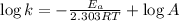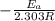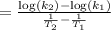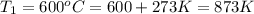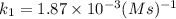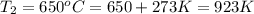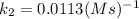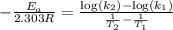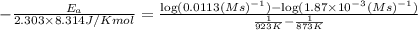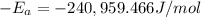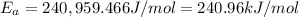The second-order rate constant for the decomposition of nitrous oxide to nitrogen molecules and oxygen atoms has been determined at various temperatures:
k(1/M·s) t(°C)
1.87 x10^-3 600
0.0113 650
0.0569 700
0.244 750
Determining the activation energy graphically.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 08:00
Nconcentration refers to the molar concentration of an ion in solution. it may be identical to, or greater or less than, the molar concentration of the compound containing the ion that was used to make the solution. for soluble salts, the molarity of a particular ion is equal to the molarity of that compound times the subscript for that ion. for example, 1 m of alcl3 is 1 m in al3+ and 3 m in cl−. 1 m of (nh4)2so4 is 2 m in nh4+ and 1 m in so42−. part a what is the concentration of k+ in 0.15 m of k2s? view available hint(s) nothing m m part b if cacl2 is dissolved in water, what can be said about the concentration of the ca2+ ion? view available hint(s) if is dissolved in water, what can be said about the concentration of the ion? it has the same concentration as the cl− ion. its concentration is half that of the cl− ion. its concentration is twice that of the cl− ion. its concentration is one-third that of the cl− ion. part c a scientist wants to make a solution of tribasic sodium phosphate, na3po4, for a laboratory experiment. how many grams of na3po4 will be needed to produce 550 ml of a solution that has a concentration of na+ ions of 0.700 m ? express your answer numerically in grams. view available hint(s) mass of na3po4 n a 3 p o 4 = nothing g provide feedback
Answers: 3

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
The second-order rate constant for the decomposition of nitrous oxide to nitrogen molecules and oxyg...
Questions

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

English, 10.09.2020 01:01

English, 10.09.2020 01:01

History, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

Social Studies, 10.09.2020 01:01

English, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

English, 10.09.2020 01:01

Physics, 10.09.2020 01:01

Mathematics, 10.09.2020 01:01

English, 10.09.2020 01:01

History, 10.09.2020 01:01