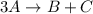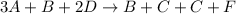Chemistry, 28.02.2020 02:26 cjjjjjjjjjjjjj
Analyzing a new reaction
Consider the following elementary steps that make up the mechanism of a certain reaction:
3A→B+C
B+2D→C+F
1. What is the overall reaction?
2. What is the rate law for step one of this reaction?
3. What is the rate law for step two of this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
Analyzing a new reaction
Consider the following elementary steps that make up the mechanism of...
Consider the following elementary steps that make up the mechanism of...
Questions


Mathematics, 03.01.2022 01:00



Mathematics, 03.01.2022 01:00

Mathematics, 03.01.2022 01:00

Geography, 03.01.2022 01:00

Geography, 03.01.2022 01:00

History, 03.01.2022 01:00


Mathematics, 03.01.2022 01:00

Arts, 03.01.2022 01:00


Health, 03.01.2022 01:00

Mathematics, 03.01.2022 01:00

World Languages, 03.01.2022 01:00

English, 03.01.2022 01:00

English, 03.01.2022 01:00


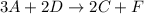
![\text{Rate}=k[A]^3](/tpl/images/0527/6240/c9f9f.png)
![\text{Rate}=k[B][D]^2](/tpl/images/0527/6240/1d2cc.png)
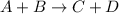
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0527/6240/10aeb.png)
![[A]](/tpl/images/0527/6240/6aa06.png) and
and ![[B]](/tpl/images/0527/6240/db909.png) = concentration of A and B reactant
= concentration of A and B reactant