

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
What is the rate law for the following mechanism in terms of the overall rate constant k? Step1:Step...
Questions

Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10


Mathematics, 25.01.2021 16:10


English, 25.01.2021 16:10




Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10

Engineering, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10

Mathematics, 25.01.2021 16:10



![Rate=k'[A][B]^2](/tpl/images/0527/6198/02776.png)
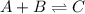 (fast)
(fast) (slow)
(slow)![Rate=k[B][C]](/tpl/images/0527/6198/2d91f.png) ............(1)
............(1)![K=\frac{[C]}{[A][B]}](/tpl/images/0527/6198/f1e20.png)
![[C]=K\times [A][B]](/tpl/images/0527/6198/91a27.png) ...........(2)
...........(2)![Rate=k[B]\times K\times [A][B]](/tpl/images/0527/6198/d3849.png)
![Rate=K\times k[A][B]^2](/tpl/images/0527/6198/56d48.png)


