Chemistry, 28.02.2020 03:21 hnsanders00
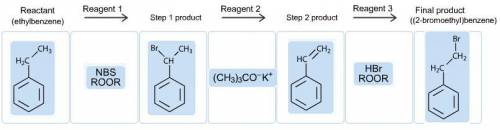
"Construct a multistep synthetic route from ethylbenzene to (2-bromoethyl)benzene by dragging the appropriate items into the bins. Note that each bin will hold only one item, and not all reagents and structures will be used."

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
"Construct a multistep synthetic route from ethylbenzene to (2-bromoethyl)benzene by dragging the ap...
Questions

Mathematics, 18.09.2019 18:00

Business, 18.09.2019 18:00


Health, 18.09.2019 18:00



Computers and Technology, 18.09.2019 18:00



History, 18.09.2019 18:00



Mathematics, 18.09.2019 18:00

Social Studies, 18.09.2019 18:00

Mathematics, 18.09.2019 18:00

Mathematics, 18.09.2019 18:00



Mathematics, 18.09.2019 18:00