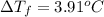Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
a. If the freezing point of the water–glycol solution is −14.0 °C, what mass of must have been added?
b. What is the boiling point of the coolant mixture? Kb(H20) = 0.52 degrees celcius kg mol^-1.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
Questions


Computers and Technology, 12.10.2020 20:01



Social Studies, 12.10.2020 20:01


Spanish, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01

Mathematics, 12.10.2020 20:01



Chemistry, 12.10.2020 20:01


History, 12.10.2020 20:01

Advanced Placement (AP), 12.10.2020 20:01



Geography, 12.10.2020 20:01



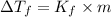
 = freezing point of solution
= freezing point of solution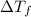 =depression in freezing point
=depression in freezing point 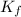 = freezing point constant
= freezing point constant 


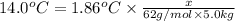

 =elevation in boiling point =
=elevation in boiling point =  = boiling point constant
= boiling point constant