Will mark brainliest if solved correctly.
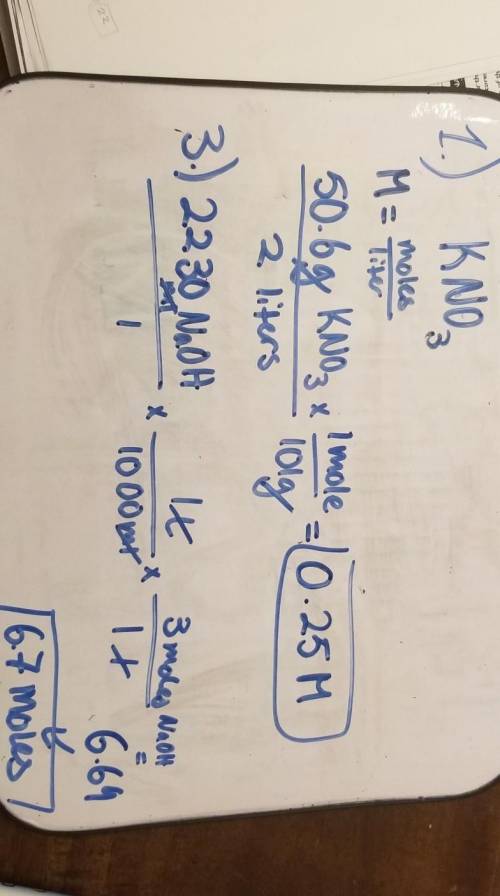
What is the concentration of a solution of KNO3 (mol...

Chemistry, 28.02.2020 03:59 zaheema786ovxirt
Will mark brainliest if solved correctly.
What is the concentration of a solution of KNO3 (molecular mass=101 g/mole) that contains 50.6 g of KNO3 in 2.00 liters of the solution?
25.25 M
0.500 M
2.00 M
0.25 M
3.
What is the total number of moles in 2230 mL of 3.0 M NaOH solution?
1.5 moles
6.7 moles
3.0 moles
0.743 moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Questions




Mathematics, 24.08.2019 17:10

History, 24.08.2019 17:10




History, 24.08.2019 17:20


Mathematics, 24.08.2019 17:20

Geography, 24.08.2019 17:20

Social Studies, 24.08.2019 17:20


Mathematics, 24.08.2019 17:20


Mathematics, 24.08.2019 17:20

Biology, 24.08.2019 17:20

Mathematics, 24.08.2019 17:20