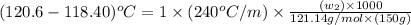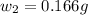A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-240 С kg-mol-1. A solution is prepared by dissolving some benzamide (C, H,NO) in 150. g of X. This solution boils at 120.6 C. Calculate the mass of C, H,NO that was dissolved Be sure your answer is rounded to the correct number of significiant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


You know the right answer?
A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-...
Questions

Mathematics, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30


English, 15.07.2019 07:30

Mathematics, 15.07.2019 07:30









History, 15.07.2019 07:30



Mathematics, 15.07.2019 07:30


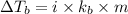
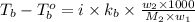
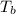 = boiling point of solution =
= boiling point of solution = 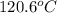
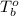 = boiling point of liquid X =
= boiling point of liquid X = 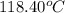
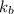 = boiling point constant of liquid X =
= boiling point constant of liquid X = 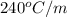
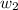 = mass of solute (benzamide ) = ?
= mass of solute (benzamide ) = ?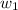 = mass of solvent (liquid X) = 150 g
= mass of solvent (liquid X) = 150 g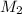 = molar mass of solute (benzamide ) = 121.14 g/mol
= molar mass of solute (benzamide ) = 121.14 g/mol