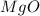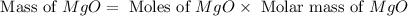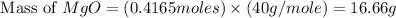Chemistry, 28.02.2020 19:57 stinematesa
You may want to reference (Page) section 4.3 while completing this problem. Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. The balanced equation for the reaction is 2Mg(s)+O2(g)→2MgO(s) 2 M g ( s ) + O 2 ( g ) → 2 M g O ( s ) When 10.0 g g Mg M g is allowed to react with 10.6 g g O2 O 2 , 12.1 g g MgO Mg O is collected.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

You know the right answer?
You may want to reference (Page) section 4.3 while completing this problem. Magnesium oxide can be m...
Questions

Chemistry, 11.09.2019 23:30



Mathematics, 11.09.2019 23:30


History, 11.09.2019 23:30


Mathematics, 11.09.2019 23:30



History, 11.09.2019 23:30






Health, 11.09.2019 23:30


English, 11.09.2019 23:30

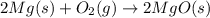
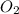 = 10.6 g
= 10.6 g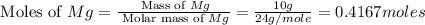
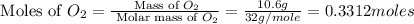
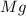 react with 1 mole of
react with 1 mole of 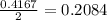 moles of
moles of