
Chemistry, 28.02.2020 19:13 mrhortert9348
We want to calculate the concentrations of all species in a 0.58 M Na 2 SO 3 (sodium sulfite) solution. The ionization constants for sulfurous acid are K a1 = 1.4 × 10 − 2 and K a2 = 6.3 × 10 − 8 . Let's start by first calculating the concentration of the spectator ion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
We want to calculate the concentrations of all species in a 0.58 M Na 2 SO 3 (sodium sulfite) soluti...
Questions

Mathematics, 24.01.2022 23:20




Mathematics, 24.01.2022 23:20


Mathematics, 24.01.2022 23:20

History, 24.01.2022 23:20


English, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20


Mathematics, 24.01.2022 23:20


Chemistry, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Computers and Technology, 24.01.2022 23:20

English, 24.01.2022 23:20

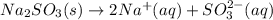
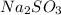 produces 2 moles of cations.
produces 2 moles of cations.![[Na^{+}] = 2[Na_{2}SO_{3}] = 2 \times 0.58](/tpl/images/0528/1243/dd619.png)
![[SO^{2-}_{3}] = [Na_{2}SO_{3}]](/tpl/images/0528/1243/7c50d.png) = 0.58 M
= 0.58 M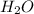 to form
to form 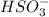 and
and 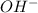 .
. 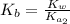
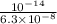
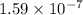
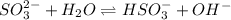
![K_{b} = \frac{[HSO^{-}_{3}][OH^{-}]}{[SO^{2-}_{3}]}](/tpl/images/0528/1243/228db.png)
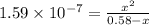
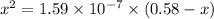
![[HSO^{-}_{3}] = [OH^{-}]](/tpl/images/0528/1243/11935.png) = 0.0003 M
= 0.0003 M![[SO^{2-}_{3}]](/tpl/images/0528/1243/4e810.png) = 0.58 - 0.0003
= 0.58 - 0.0003![[HSO^{-}_{3}]](/tpl/images/0528/1243/3fe07.png) = 0.0003 M
= 0.0003 M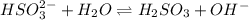
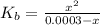
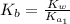
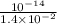
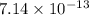
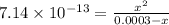
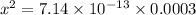
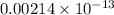
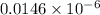
![[OH^{-}] = [H_{2}SO_{3}]](/tpl/images/0528/1243/01edf.png) =
= 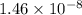
![[H^{+}] = \frac{10^{-14}}{[OH^{-}]}](/tpl/images/0528/1243/fae45.png)
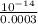
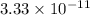 M
M

