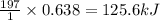Consider the combination reaction between sulfur dioxide gas and oxygen gas to produce sulfur trioxide. Also consider that you have 40.86 g of sulfur dioxide gas and 40.01 g of oxygen gas. For this reaction, use LaTeX: \Delta Δ Hrxn = - 197 kJ/mol of product. If the reaction proceeds to completion, what is the maximum amount of heat (in kJ) that you could expect to generate?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Consider the combination reaction between sulfur dioxide gas and oxygen gas to produce sulfur trioxi...
Questions

Mathematics, 06.07.2021 22:10


Mathematics, 06.07.2021 22:10


Social Studies, 06.07.2021 22:10




Mathematics, 06.07.2021 22:10

Mathematics, 06.07.2021 22:10



Chemistry, 06.07.2021 22:10




Computers and Technology, 06.07.2021 22:10

Computers and Technology, 06.07.2021 22:10


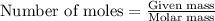 .....(1)
.....(1)
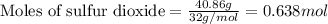
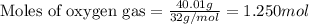
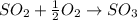
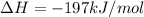
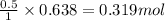 of oxygen gas
of oxygen gas