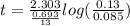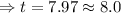Chemistry, 28.02.2020 19:21 semajac11135
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. 10. 11 7.0 12 8.0

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions



Arts, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10



Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

History, 01.06.2021 20:10

English, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10


Mathematics, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10

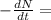 λN
λN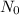 t time t = 0.
t time t = 0.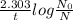
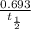
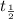 = 13 min
= 13 min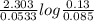
![\textrm{rate of reaction}=-\frac{d[A]}{dt} =k[A]](/tpl/images/0528/1630/bb97d.png)
![k=\frac{2.303}{t} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/263ab.png)
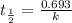
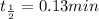
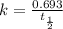
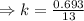
![\Rightarrow t=\frac{2.303}{k} log\frac{[A_0]}{[A]}](/tpl/images/0528/1630/3dc05.png)
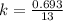 , [A₀] = 0.13 m and [ A] = 0.085 M
, [A₀] = 0.13 m and [ A] = 0.085 M