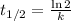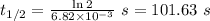Chemistry, 28.02.2020 19:45 brianadee800
The first-order rate constant for the decomposition of N2O5, 2N2O5(g)→4NO2(g)+O2(g) at 70∘C is 6.82×10−3 s−1. Starting with 8.00×10−2 mol of N2O5(g) in a volume of 2.9 L, how many moles of reactant are left after 5 minutes? What is its half-life?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1
You know the right answer?
The first-order rate constant for the decomposition of N2O5, 2N2O5(g)→4NO2(g)+O2(g) at 70∘C is 6.82×...
Questions

Mathematics, 18.08.2019 15:00




Physics, 18.08.2019 15:00

Mathematics, 18.08.2019 15:00

Mathematics, 18.08.2019 15:00


Physics, 18.08.2019 15:00

Mathematics, 18.08.2019 15:00


Mathematics, 18.08.2019 15:00


Mathematics, 18.08.2019 15:00



Mathematics, 18.08.2019 15:00

History, 18.08.2019 15:00


English, 18.08.2019 15:00

![[A_t]=[A_0]e^{-kt}](/tpl/images/0528/2808/1ef89.png)
![[A_t]](/tpl/images/0528/2808/5262c.png) is the concentration at time t
is the concentration at time t ![[A_0]](/tpl/images/0528/2808/9a686.png) is the initial concentration =
is the initial concentration = 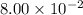 mol
mol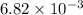 s⁻¹
s⁻¹![[A_t]=8.00\times 10^{-2}e^{-6.82\times 10^{-3}\times 300}\ mol=0.01034\ mol](/tpl/images/0528/2808/69780.png)