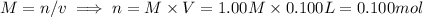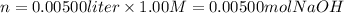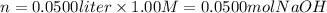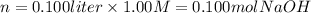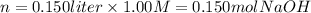Chemistry, 28.02.2020 19:46 robert7248
A) A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the following quantities of 1.00 M NaOH to the reaction flask. Classify the following conditions after the equivalence point. Write down the items to their respective table.
i) 5.00 mL of 1.00 M NaOH
ii) 50.0 mL of 1.00 M NaOH
iii) 100 mL of 1.00 M NaOH
iv) 150 mL of 1.00 M NaOH
v) 200 mL of 1.00 M NaOH
Before equivalence point At equivalence point After equivalence point

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
A) A volume of 100 mL of 1.00 M HCl solution is titrated with 1.00 M NaOH solution. You added the fo...
Questions

Mathematics, 13.01.2021 15:50



English, 13.01.2021 15:50

Business, 13.01.2021 15:50


Arts, 13.01.2021 15:50



Computers and Technology, 13.01.2021 15:50


Mathematics, 13.01.2021 15:50

Mathematics, 13.01.2021 15:50

Mathematics, 13.01.2021 15:50



Mathematics, 13.01.2021 15:50