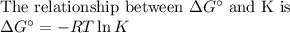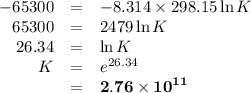Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Sing any data you can find in the ALEKS Data resource, calculate the equilibrium constant K at 25.0°...
Questions

Business, 25.04.2021 09:00

Mathematics, 25.04.2021 09:00




History, 25.04.2021 09:00


English, 25.04.2021 09:00

Mathematics, 25.04.2021 09:00

Biology, 25.04.2021 09:00


English, 25.04.2021 09:00


Computers and Technology, 25.04.2021 09:00


Business, 25.04.2021 09:00

Engineering, 25.04.2021 09:00

Physics, 25.04.2021 09:00


Mathematics, 25.04.2021 09:00