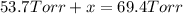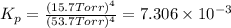Chemistry, 28.02.2020 22:46 AutumnJoy12
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressure of water vapor is 53.7 torr, the total pressure at equilibrium is 69.4 torr. Calculate Kp for this reaction at 1181 K. 3 Fe(s) + 4 H2O(g) equilibrium reaction arrow Fe3O4(s) + 4 H2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressu...
Questions



Mathematics, 30.08.2019 08:50

Mathematics, 30.08.2019 08:50

Mathematics, 30.08.2019 08:50

Mathematics, 30.08.2019 08:50



Biology, 30.08.2019 08:50



Chemistry, 30.08.2019 08:50

History, 30.08.2019 08:50

Mathematics, 30.08.2019 08:50



Mathematics, 30.08.2019 08:50



Mathematics, 30.08.2019 08:50

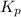 for this reaction at 1181 K is
for this reaction at 1181 K is  .
.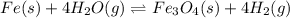
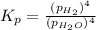
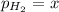
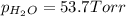
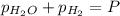 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)