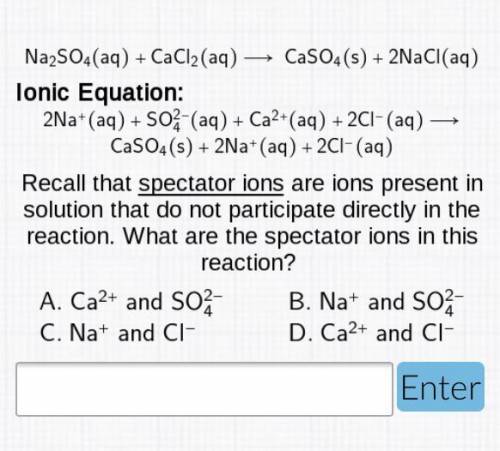
Na2SO4(aq)+CaCl2(aq)—>CaSO4(s)+2 NaCl(aq)
Ionic Equation: 2Na+(aq)+SO2/4-(aq)+Ca2+(aq)+2Cl-...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
Questions


Mathematics, 24.11.2020 01:00

English, 24.11.2020 01:00

English, 24.11.2020 01:00




Computers and Technology, 24.11.2020 01:00


Mathematics, 24.11.2020 01:00


English, 24.11.2020 01:00

Computers and Technology, 24.11.2020 01:00


World Languages, 24.11.2020 01:00


Law, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00