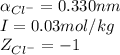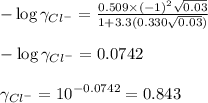Chemistry, 29.02.2020 00:27 haleyrene2663
For a solution comprised of PbSO4 (0.025 mol/kg) and MgCl2 (0.01 mol/kg) determine (19 pts) (a) the ionic strength (5 pts) (b) activity coefficients of each of these ions in solution (6 pts)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
For a solution comprised of PbSO4 (0.025 mol/kg) and MgCl2 (0.01 mol/kg) determine (19 pts) (a) the...
Questions






Computers and Technology, 23.12.2019 20:31








Chemistry, 23.12.2019 20:31




Computers and Technology, 23.12.2019 20:31

Computers and Technology, 23.12.2019 20:31

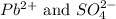 ions in lead sulfate solution is 0.352 and 0.324 respectively and the activity coefficient of
ions in lead sulfate solution is 0.352 and 0.324 respectively and the activity coefficient of 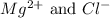 ions in magnesium chlorirde solution is 0.500 and 0.843 respectively
ions in magnesium chlorirde solution is 0.500 and 0.843 respectively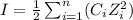
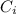 = concentration of i-th ions.
= concentration of i-th ions.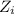 = charge of i-th ions.
= charge of i-th ions.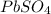 solution:
solution: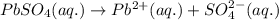
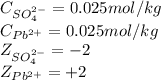
![I=\frac{1}{2}[(0.025\times (+2)^2)+(0.025\times (-2)^2)]\\\\I=0.1mol/kg](/tpl/images/0528/7729/f6f2f.png)
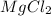 solution:
solution: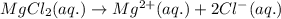
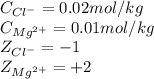
![I=\frac{1}{2}[(0.01\times (+2)^2)+(0.02\times (-1)^2)]\\\\I=0.03mol/kg](/tpl/images/0528/7729/b3f42.png)
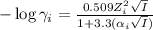
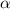 = effective hydrated radius of i-th specie (in nm)
= effective hydrated radius of i-th specie (in nm)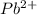 ions:
ions: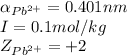
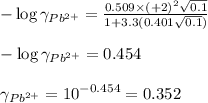
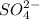 ions:
ions: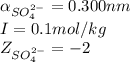
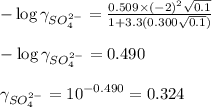
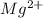 ions:
ions: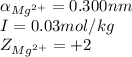
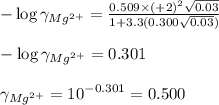
 ions:
ions: