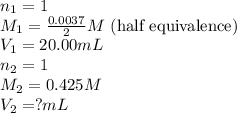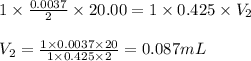Chemistry, 29.02.2020 00:24 DallasPotts7018
Find how many milliliters of NaOH should be used to reach the half-equivalence point during the titration of 20.00 mL 0.888 M butanoic acid, CH3CH2CH2COOH (Ka = 1.54×10–5) with 0.425 M NaOH solution. Enter 2 decimal places.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Find how many milliliters of NaOH should be used to reach the half-equivalence point during the titr...
Questions

English, 28.06.2019 12:30


English, 28.06.2019 12:30



Mathematics, 28.06.2019 12:30


Biology, 28.06.2019 12:30


Mathematics, 28.06.2019 12:30







History, 28.06.2019 12:30

Physics, 28.06.2019 12:30


History, 28.06.2019 12:30

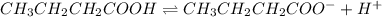
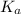 for above equation follows:
for above equation follows:![K_a=\frac{[CH_3CH_2CH_2COO^-][H^+]}{[CH_3CH_2CH_2COOH]}](/tpl/images/0528/7620/1b477.png)
![[CH_3CH_2CH_2COOH]=0.888M\\K_a=1.54\times 10^{-5}](/tpl/images/0528/7620/c91e2.png)
![[CH_3CH_2CH_2COO^-]=[H^+]](/tpl/images/0528/7620/548e3.png)
![1.54\times 10^{-5}=\frac{[H^+]^2}{0.888}](/tpl/images/0528/7620/5816d.png)
![[H^+]=-0.0037,0.0037](/tpl/images/0528/7620/74915.png)
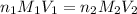
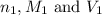 are the n-factor, molarity and volume of acid which is butanoic acid
are the n-factor, molarity and volume of acid which is butanoic acid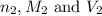 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.