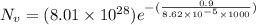Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Calculate the equilibrium number of vacancies per cubic meter for copper at 1000K. The energy for va...
Questions




History, 20.09.2020 14:01




Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01

Health, 20.09.2020 14:01






History, 20.09.2020 14:01

History, 20.09.2020 14:01



Health, 20.09.2020 14:01

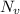 .
.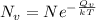
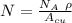
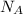 = Avogadro Number = 6.023×10²³
= Avogadro Number = 6.023×10²³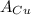 = 63.5 g/mole
= 63.5 g/mole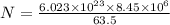
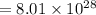 g/mole
g/mole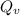 =0.9 ev/atom , T= 1000k
=0.9 ev/atom , T= 1000k