
Chemistry, 29.02.2020 01:51 heyysiirr3064
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily increases, but that of O is less than that of N. The best interpretation of the lowervalue for O is that:1. the ionization potential of N is a maximum and the values decrease steadily for the elements O, F, and Ne.2. there is more shielding of the nuclear charge in O than in B, C, or N.3. the electron removed from O is farther from the nucleus and therefore less tightly bound than that in N.4. the electron removed from O corresponds to a different value of the quantum number L than that of the electron removed from B, C, or N.5. the half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily inc...
Questions

Chemistry, 31.12.2020 05:30






Mathematics, 31.12.2020 05:30


Computers and Technology, 31.12.2020 05:30






Mathematics, 31.12.2020 05:30

Physics, 31.12.2020 05:30



History, 31.12.2020 05:30

Biology, 31.12.2020 05:30

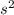 2
2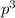 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.

