Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Calculate the cell potential E at 25°C for the reaction 2 Al(s) + 3 Fe2+(aq) → 2 Al3+(aq) + 3 Fe(s)...
Questions

Mathematics, 14.05.2021 14:30



Mathematics, 14.05.2021 14:30




Mathematics, 14.05.2021 14:30



Mathematics, 14.05.2021 14:30


Mathematics, 14.05.2021 14:30

Advanced Placement (AP), 14.05.2021 14:30

Mathematics, 14.05.2021 14:30



History, 14.05.2021 14:40

Geography, 14.05.2021 14:40

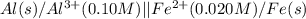
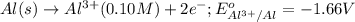
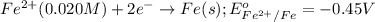
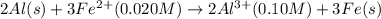
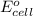 of the reaction, we use the equation:
of the reaction, we use the equation:
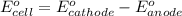
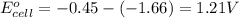
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Al^{3+}]^2}{[Fe^{2+}]^3}](/tpl/images/0528/8715/94ca6.png)
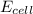 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V