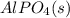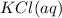Chemistry, 29.02.2020 03:29 gisellekarime
Aqueous potassium phosphate reacts with aqueous aluminum chloride to form aqueous potassium chloride and solid aluminum phosphate. Write the balanced chemical equation for the reaction. Include physical states.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Aqueous potassium phosphate reacts with aqueous aluminum chloride to form aqueous potassium chloride...
Questions

Mathematics, 24.01.2021 20:20

Chemistry, 24.01.2021 20:20

Health, 24.01.2021 20:20

English, 24.01.2021 20:20


Mathematics, 24.01.2021 20:20


Mathematics, 24.01.2021 20:20

Health, 24.01.2021 20:20


Mathematics, 24.01.2021 20:20


Advanced Placement (AP), 24.01.2021 20:20


Mathematics, 24.01.2021 20:20


Mathematics, 24.01.2021 20:20



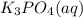 +
+ 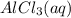 --->
---> 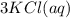 +
+