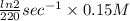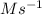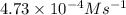Chemistry, 29.02.2020 03:23 tobywaffle1234
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces a plot of Ln[(CH3)3CCl] vs. time that is linear with a negative slope. Suppose the reaction is carried out under conditions such that the half-life of the reaction is 2.20 x 102 s. What is the instantaneous rate of reaction when [(CH3)3CCl] = 0.15 M?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces...
Questions

English, 06.05.2020 20:07



Mathematics, 06.05.2020 20:07



English, 06.05.2020 20:07




Mathematics, 06.05.2020 20:07

Mathematics, 06.05.2020 20:07

Chemistry, 06.05.2020 20:07






English, 06.05.2020 20:07

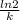
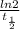
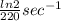
![[(CH_{3})_{3}CCl]^{1}](/tpl/images/0528/9812/8a6e5.png)