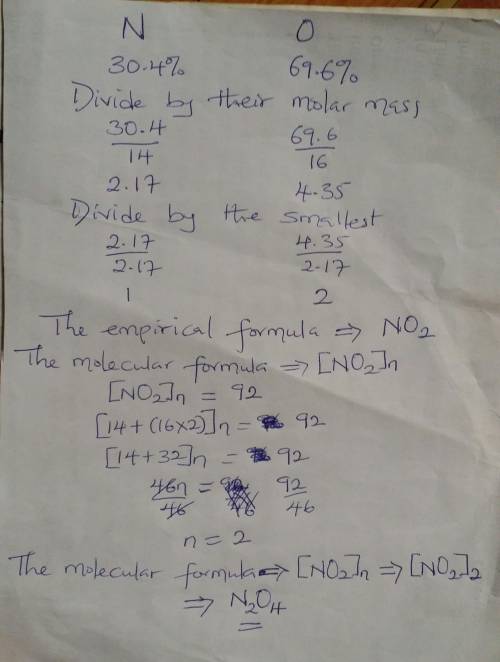Extension Questions:
17. An oxide of nitrogen is found to contain 69.6% oxygen and has a molar...

Chemistry, 29.02.2020 07:40 isabelperez063
Extension Questions:
17. An oxide of nitrogen is found to contain 69.6% oxygen and has a molar mass of 92.0 g/mole.
a. What is the % nitrogen in this compound?
b. Find the empirical formula and molecular formula for this compound.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Questions


Mathematics, 24.03.2021 01:00




English, 24.03.2021 01:00


English, 24.03.2021 01:00

Advanced Placement (AP), 24.03.2021 01:00

Biology, 24.03.2021 01:00

Social Studies, 24.03.2021 01:00




Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00