Chemistry, 01.03.2020 00:17 2022maldonadoleonel
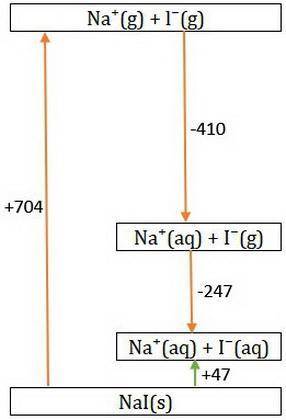
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+= -410.0 kJ/mol
AHhydr of -= -247 kJ/mol
What is the AHSol of this compound? Use AHsol = -AHlat + AHhydr.
0-867 kJ/mol
|-867.0 kJ/mol
0 47 kJ/mol
0 47.0 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

You know the right answer?
Consider Nal → Na+ + - and the following information.
Hlat = -704 kJ/mol
AHhydr of Na+=...
Hlat = -704 kJ/mol
AHhydr of Na+=...
Questions



Spanish, 21.01.2020 19:31






Medicine, 21.01.2020 19:31

Medicine, 21.01.2020 19:31








Computers and Technology, 21.01.2020 19:31