freezing and condensation


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
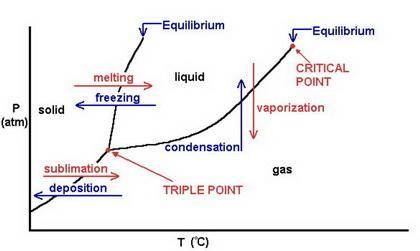
Which of these processes take place at the same temperature?
freezing and condensation
freezing and condensation
Questions

Engineering, 01.12.2020 23:30

English, 01.12.2020 23:30

Chemistry, 01.12.2020 23:30


English, 01.12.2020 23:30

Mathematics, 01.12.2020 23:30


Health, 01.12.2020 23:30

Computers and Technology, 01.12.2020 23:30

History, 01.12.2020 23:30


Biology, 01.12.2020 23:30



Mathematics, 01.12.2020 23:30

Mathematics, 01.12.2020 23:30

Mathematics, 01.12.2020 23:30

Mathematics, 01.12.2020 23:30

Computers and Technology, 01.12.2020 23:30