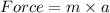4 Points
A jar of mass 2.1 kg sits on a table. What is the normal force acting on the
jar?...

Chemistry, 01.03.2020 06:06 22katelynfrankouqqrb
4 Points
A jar of mass 2.1 kg sits on a table. What is the normal force acting on the
jar?
O
A. 33.1 N.
B. 2.1 N
O C. 5.3 N
O D. 20.6N
SUBME

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

You know the right answer?
Questions

History, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10


Mathematics, 09.12.2020 08:10


History, 09.12.2020 08:10



Physics, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10

Social Studies, 09.12.2020 08:10

English, 09.12.2020 08:10




Mathematics, 09.12.2020 08:10



Mathematics, 09.12.2020 08:10

Mathematics, 09.12.2020 08:10