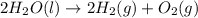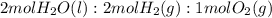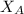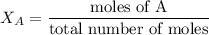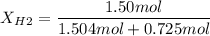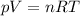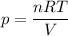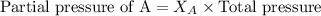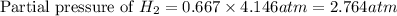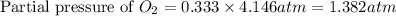Chemistry, 01.03.2020 18:38 taegibbby03
Electrolysis is a process that can split water into hydrogen gas and oxygen gas. If 27.1 g of water are split, what is the resulting mole fraction of hydrogen gas? Of oxygen gas? If the gases are in a 12.2 L container at standard temperature, what is the partial pressure of the hydrogen gas? Of oxygen gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1


Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

You know the right answer?
Electrolysis is a process that can split water into hydrogen gas and oxygen gas. If 27.1 g of water...
Questions


Chemistry, 10.01.2020 06:31





Mathematics, 10.01.2020 06:31



History, 10.01.2020 06:31

History, 10.01.2020 06:31



History, 10.01.2020 06:31

Chemistry, 10.01.2020 06:31


History, 10.01.2020 06:31

Mathematics, 10.01.2020 06:31


Geography, 10.01.2020 06:31