

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2
You know the right answer?
A molecule has the empirical formula C4H6O. If its molecular weight is determined to be about 212 g/...
Questions



Mathematics, 18.05.2020 07:57





Mathematics, 18.05.2020 07:57

Spanish, 18.05.2020 07:57



Mathematics, 18.05.2020 08:57

Mathematics, 18.05.2020 08:57



Mathematics, 18.05.2020 08:57



Mathematics, 18.05.2020 08:57

History, 18.05.2020 08:57

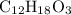 .
. 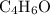 , then the molecular formula would be
, then the molecular formula would be 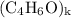 , or equivalently
, or equivalently 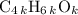 , where
, where 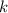 is a positive whole number (
is a positive whole number (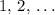 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of 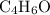 would be
would be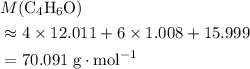 .
.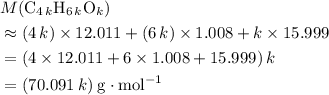 .
.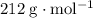 ,
, 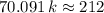 .
.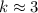 . (Round to the nearest whole number.)
. (Round to the nearest whole number.)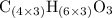 , which simplifies to
, which simplifies to 

