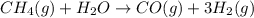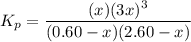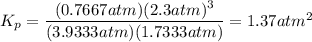Chemistry, 02.03.2020 16:51 jonestheproblem5029
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a flask with of methane gas and of water vapor at . She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be . Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

You know the right answer?
Steam reforming of methane ( ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrog...
Questions

History, 31.08.2020 17:01

History, 31.08.2020 17:01

History, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

English, 31.08.2020 17:01

Chemistry, 31.08.2020 17:01

History, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01

Social Studies, 31.08.2020 17:01


Biology, 31.08.2020 17:01

English, 31.08.2020 17:01


Mathematics, 31.08.2020 17:01

Mathematics, 31.08.2020 17:01


English, 31.08.2020 17:01

Biology, 31.08.2020 17:01