
Chemistry, 02.03.2020 16:50 jameslinimk
Calculate E o , E, and ΔG for the following cell reactions (a) Mg(s) + Sn2+(aq) ⇌ Mg2+(aq) + Sn(s) where [Mg2+] = 0.025 M and [Sn2+] = 0.040 M E o = V E = V ΔG = kJ (b) 3Zn(s) + 2Cr3+(aq) ⇌ 3Zn2+(aq) + 2Cr(s) where [Cr3+] = 0.040 M and [Zn2+] = 0.0085 M E o = V E = V ΔG = kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Calculate E o , E, and ΔG for the following cell reactions (a) Mg(s) + Sn2+(aq) ⇌ Mg2+(aq) + Sn(s) w...
Questions


Mathematics, 24.09.2020 21:01




Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01


Mathematics, 24.09.2020 21:01


Mathematics, 24.09.2020 21:01




Social Studies, 24.09.2020 21:01



History, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01

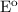 = 2.23 V, E = 2.2 V,
= 2.23 V, E = 2.2 V, 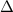 G = 424.6 kJ/mol
G = 424.6 kJ/mol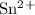 = -0.14
= -0.14 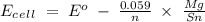

 96500
96500 



