
Chemistry, 02.03.2020 16:56 raynaesquivel
A reaction mixture initially contains 0.86 atm NO and 0.86 atm SO3. Determine the equilibrium pressure of NO2 if Kp for the reaction at this temperature is 0.0118. NO(g) SO3(g) NO2(g) SO2(g)

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1

Chemistry, 23.06.2019 13:00
What mass of ca(oh)2 is needed to make 1250ml of a .75m solution?
Answers: 3

You know the right answer?
A reaction mixture initially contains 0.86 atm NO and 0.86 atm SO3. Determine the equilibrium pressu...
Questions


Mathematics, 23.10.2021 20:20



Computers and Technology, 23.10.2021 20:20

Biology, 23.10.2021 20:20




Mathematics, 23.10.2021 20:20

Social Studies, 23.10.2021 20:20







Social Studies, 23.10.2021 20:20

Health, 23.10.2021 20:20

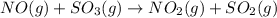
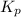 = 0.0118.
= 0.0118.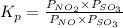
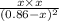
 is 0.084 atm.
is 0.084 atm.



