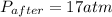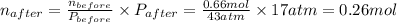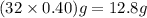A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from the tank to lower the pressure of the gas from 43 atm to 17 atm. Assume that the volume of the tank and the temperature of the oxygen are constant during this operation. Answer in units of g.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1

Chemistry, 23.06.2019 11:50
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1

You know the right answer?
A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from t...
Questions




Computers and Technology, 20.11.2020 21:10

Mathematics, 20.11.2020 21:10



Health, 20.11.2020 21:10



Arts, 20.11.2020 21:10


History, 20.11.2020 21:10

Health, 20.11.2020 21:10


History, 20.11.2020 21:10

Mathematics, 20.11.2020 21:10

Mathematics, 20.11.2020 21:10

Chemistry, 20.11.2020 21:10

Biology, 20.11.2020 21:10

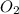 must be withdrawn from tank
must be withdrawn from tank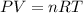
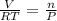
 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of 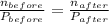
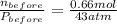 and
and