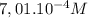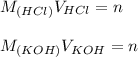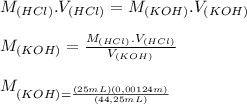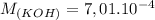Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
A student has a 0.00124 M HCl (aq) solution and she titrates 25.00 mL of this solution against an un...
Questions

Mathematics, 20.04.2021 22:50

History, 20.04.2021 22:50

Social Studies, 20.04.2021 22:50

History, 20.04.2021 22:50


English, 20.04.2021 22:50


Mathematics, 20.04.2021 22:50

Biology, 20.04.2021 22:50


English, 20.04.2021 22:50

Chemistry, 20.04.2021 22:50





Mathematics, 20.04.2021 22:50

Mathematics, 20.04.2021 22:50

Advanced Placement (AP), 20.04.2021 22:50