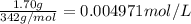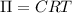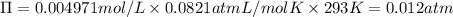Chemistry, 02.03.2020 16:58 gjeaneasley
Wastewater discharged into a stream by a sugar refinery contains 1.70 g of sucrose (C12H22O11) per liter. A government-industry project is designed to test the feasibility of removing the sugar by reverse osmosis. What pressure must be applied to the apparatus at 20.°C to produce pure water?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Wastewater discharged into a stream by a sugar refinery contains 1.70 g of sucrose (C12H22O11) per l...
Questions


Computers and Technology, 24.11.2021 01:20




Chemistry, 24.11.2021 01:20

Mathematics, 24.11.2021 01:20



English, 24.11.2021 01:20



Mathematics, 24.11.2021 01:20

Mathematics, 24.11.2021 01:20


Mathematics, 24.11.2021 01:20




English, 24.11.2021 01:20