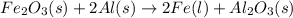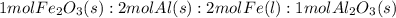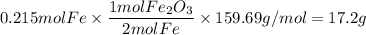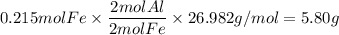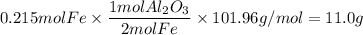Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is given below. Fe2O3(s) + 2 Al(s) 2 Fe(l) + Al2O3(s) What masses of iron(III) oxide and aluminum must be used to produce 12.0 g iron? iron (III) oxide WebAssign will check your answer for the correct number of significant figures. 17.2 Correct: Your answer is correct. g aluminum WebAssign will check your answer for the correct number of significant figures. 2.98 Incorrect: Your answer is incorrect. g What is the maximum mass of aluminum oxide that could be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3


Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs,...
Questions



Mathematics, 02.07.2019 08:30

History, 02.07.2019 08:30

Physics, 02.07.2019 08:30




Advanced Placement (AP), 02.07.2019 08:30











Mathematics, 02.07.2019 08:30