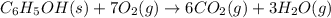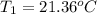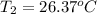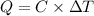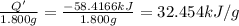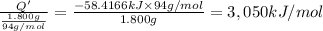A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capacity is 11.66 kJ/?C. The temperature of the calorimeter plus contents increased from 21.36?Cto 26.37?C. Part A
Write a balanced chemical equation for the bomb calorimeter reaction.
Part B:
What is the heat of combustion per gram of phenol?Part C:
Per mole of phenol?

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
12.00 moles of naclo3 will produce how many grams of o2? a) 256 g of o2 b) 576 g of o2 c) 288 g of o2
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capac...
Questions

History, 20.08.2019 16:10

English, 20.08.2019 16:10

Mathematics, 20.08.2019 16:10


Mathematics, 20.08.2019 16:10


History, 20.08.2019 16:10

Geography, 20.08.2019 16:10


Health, 20.08.2019 16:10

Mathematics, 20.08.2019 16:10

History, 20.08.2019 16:10

Health, 20.08.2019 16:10

Health, 20.08.2019 16:10

Mathematics, 20.08.2019 16:10


Mathematics, 20.08.2019 16:10

Computers and Technology, 20.08.2019 16:10


Mathematics, 20.08.2019 16:10