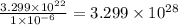Chemistry, 02.03.2020 18:11 lizzie3545
Calculate the number of atoms per cubic meter in lead. The density and atomic weight of lead are 11.35 g/cm3 and 207.2 g/mol respectively. The value of Avogadro's number is 6.022 x 1023 atoms/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Calculate the number of atoms per cubic meter in lead. The density and atomic weight of lead are 11....
Questions





Spanish, 22.06.2019 19:00



History, 22.06.2019 19:00


Geography, 22.06.2019 19:00






Geography, 22.06.2019 19:00

Mathematics, 22.06.2019 19:00

Biology, 22.06.2019 19:00


Mathematics, 22.06.2019 19:00

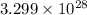
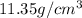 means mass of
means mass of 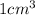 lead is 11.35 g
lead is 11.35 g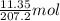 of lead = 0.05478 mol of lead
of lead = 0.05478 mol of lead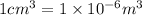
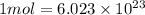 number of particles
number of particles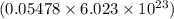 number of atoms of lead
number of atoms of lead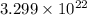 number of atoms of lead
number of atoms of lead