

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Calculate the pH and fraction of dissociation ( α ) for each of the acetic acid ( CH 3 COOH , p K a...
Questions



Geography, 02.02.2022 14:00






English, 02.02.2022 14:00



Mathematics, 02.02.2022 14:00


Chemistry, 02.02.2022 14:00



SAT, 02.02.2022 14:00


Social Studies, 02.02.2022 14:00

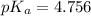
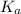
![pK_a=-\log[K_a]](/tpl/images/0530/3712/78bbf.png)
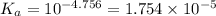
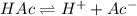
![K_a=\frac{[H^+][Ac^-]}{[HAc]}](/tpl/images/0530/3712/ce0d8.png)
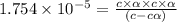
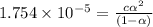
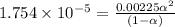
![[H^+]=c\alpha = 0.00225M\times 0.08448=0.0001901 M](/tpl/images/0530/3712/81253.png)
![pH=-\log[H^+]](/tpl/images/0530/3712/cf945.png)
![=-\log[0.0001901 M]=3.72](/tpl/images/0530/3712/5ff4a.png)


