Chemistry, 02.03.2020 18:22 hsjsjsjdjjd
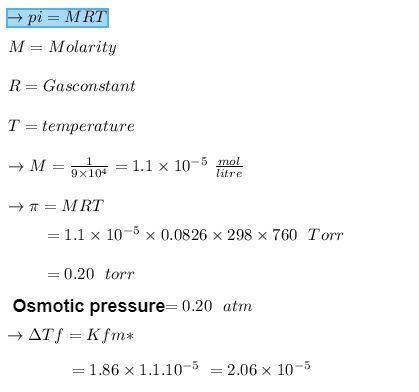
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containing 1.0 g/L of a protein (molar mass 5 9.0 3 104 g/mol) if the density of the solution is 1.0 g/cm3. b. Considering your answer to part a, which colligative property, freezing-point depression or osmotic pres- sure, would be better used to determine the molar masses of large molecules

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containi...
Questions

Social Studies, 28.08.2019 16:30

Biology, 28.08.2019 16:30

Mathematics, 28.08.2019 16:30

Computers and Technology, 28.08.2019 16:30


Mathematics, 28.08.2019 16:30

History, 28.08.2019 16:30

Computers and Technology, 28.08.2019 16:30

Mathematics, 28.08.2019 16:30





Geography, 28.08.2019 16:30



Computers and Technology, 28.08.2019 16:30



Mathematics, 28.08.2019 16:30