
Chemistry, 02.03.2020 19:06 lizzie3545
Calculate the mass of H2OH2O produced by metabolism of 2.4 kgkg of fat, assuming the fat consists entirely of tristearin (C57H110O6C57H110O6), a typical animal fat, and assuming that during metabolism, tristearin reacts with O2O2 to form only CO2CO2 and H2OH2O.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Calculate the mass of H2OH2O produced by metabolism of 2.4 kgkg of fat, assuming the fat consists en...
Questions






English, 16.04.2020 06:20



History, 16.04.2020 06:20

Mathematics, 16.04.2020 06:20

Mathematics, 16.04.2020 06:20


Mathematics, 16.04.2020 06:20


English, 16.04.2020 06:20


Physics, 16.04.2020 06:20


Business, 16.04.2020 06:20

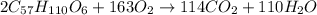
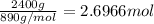
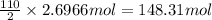 of water
of water

