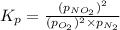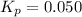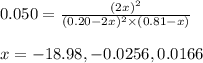Chemistry, 02.03.2020 19:27 BobBball9126
In a chamber filled with air (look up the partial pressures of nitrogen and oxygen gases) at 2200 °C, the reaction that generates nitrogen monoxide from nitrogen gas and oxygen gas has a Kp = 0.050. What are the equilibrium partial pressures of the three gases in the above reaction?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
In a chamber filled with air (look up the partial pressures of nitrogen and oxygen gases) at 2200 °C...
Questions

English, 11.09.2019 07:10

English, 11.09.2019 07:10

Mathematics, 11.09.2019 07:10


English, 11.09.2019 07:10

Mathematics, 11.09.2019 07:10

Mathematics, 11.09.2019 07:10


Chemistry, 11.09.2019 07:10



Mathematics, 11.09.2019 07:10

Geography, 11.09.2019 07:10


Mathematics, 11.09.2019 07:10

Mathematics, 11.09.2019 07:10

Physics, 11.09.2019 07:10

Geography, 11.09.2019 07:10

Engineering, 11.09.2019 07:10

Geography, 11.09.2019 07:10

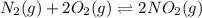
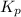 for above equation follows:
for above equation follows: