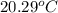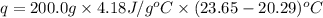A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After mixing, the temperature rises to 23.65 oC. Calculate the heat of this reaction. [assuming the density and specific heat of HBr and KOH is the same as water, 1.0 g/mL; 4.18 J/g oC, and the volume of the solution is additive].

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1


Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
A 100.0 mL of 0.500 M HBr at 20.29 oC is added to 100.0 mL of 0.500 M KOH (also at 20.29 oC). After...
Questions

Mathematics, 30.11.2020 21:00


English, 30.11.2020 21:10

Mathematics, 30.11.2020 21:10


Health, 30.11.2020 21:10


Mathematics, 30.11.2020 21:10


Mathematics, 30.11.2020 21:10



Mathematics, 30.11.2020 21:10

Mathematics, 30.11.2020 21:10



Mathematics, 30.11.2020 21:10

Health, 30.11.2020 21:10

History, 30.11.2020 21:10

English, 30.11.2020 21:10

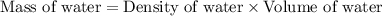
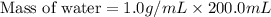
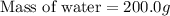
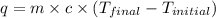
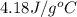
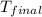 = final temperature =
= final temperature = 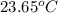
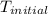 = initial temperature =
= initial temperature =