

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
The combustion of propane (C 3H 8) produces CO 2 and H 2O: C 3H 8 (g) 5O2 (g) 3CO2 (g) 4 H 2O (g) Th...
Questions

English, 27.01.2021 04:30


Mathematics, 27.01.2021 04:30

Computers and Technology, 27.01.2021 04:30

Spanish, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

Computers and Technology, 27.01.2021 04:30

Spanish, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30



Mathematics, 27.01.2021 04:30


Biology, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

English, 27.01.2021 04:30

English, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

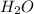 produced will be, 2 moles
produced will be, 2 moles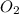 = 2.5 mol
= 2.5 mol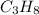 = 4.6 mol
= 4.6 mol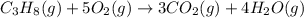
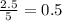 moles of
moles of 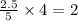 moles of
moles of 

