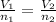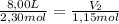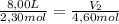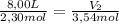Chemistry, 02.03.2020 19:49 barstr9146
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in liters, when the following changes occur in the quantity of the gas at constant pressure and temperature?a. A leak allows one-half of Ne atoms to escape. b. A sample of 3.50 mol of Ne is added to the 2.30 mol of Ne gas in the container. c. A sample of 25.0 g of Ne is added to the 2.30 mol of Ne gas in the container.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in...
Questions



Mathematics, 13.04.2021 16:50

Advanced Placement (AP), 13.04.2021 16:50


Chemistry, 13.04.2021 16:50




Arts, 13.04.2021 16:50

History, 13.04.2021 16:50


Mathematics, 13.04.2021 16:50


Engineering, 13.04.2021 16:50

Mathematics, 13.04.2021 16:50

Mathematics, 13.04.2021 16:50


English, 13.04.2021 16:50

Mathematics, 13.04.2021 16:50