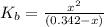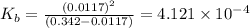Chemistry, 02.03.2020 20:38 mrmendrala
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethylamine, C2H5NH2 to be 12.067. Use the information she obtained to determine the Kb for this base.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

You know the right answer?
N the laboratory, a general chemistry student measured the pH of a 0.342 M aqueous solution of ethyl...
Questions

Geography, 13.05.2021 19:20


Computers and Technology, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20

Arts, 13.05.2021 19:20








Mathematics, 13.05.2021 19:20

Mathematics, 13.05.2021 19:20





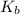 of the an ethylamine is
of the an ethylamine is  .
.![pOH=-\log[OH^-]](/tpl/images/0530/5572/fe336.png)
![1.933=-\log[OH^-]](/tpl/images/0530/5572/b301a.png)
![[OH^-]=0.0117 M](/tpl/images/0530/5572/660e1.png)

![K_b=\frac{[C_2H_5NH_3^{+}][OH^-]}{[C_2H_5NH_2]}](/tpl/images/0530/5572/63c9d.png)