Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
Consider the following thermochemical equations.
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
PCl5 (s)→PCl3 (g)+Cl2 (g) ΔH∘rxn= 87.9k...
Questions

History, 28.06.2019 07:50




History, 28.06.2019 07:50

Social Studies, 28.06.2019 07:50


Mathematics, 28.06.2019 07:50








Computers and Technology, 28.06.2019 07:50




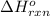 for the reaction is -749.8 kJ.
for the reaction is -749.8 kJ.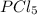 follows:
follows: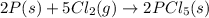
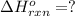
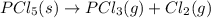
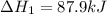 ( × 2)
( × 2)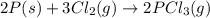
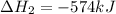
![\Delta H^o_{rxn}=[2\times (-\Delta H_1)]+[1\times \Delta H_2]](/tpl/images/0530/6491/d8429.png)